This exploration is for all ages, as the colored smilies show. You can do the hard water experiment with your whole family together!




The hard water experiment is a chemistry experiment from Solutions. Layers of Learning has hands-on experiments in every unit of this family-friendly curriculum. Learn more about Layers of Learning.
Hard water just means water that has minerals in it. The minerals usually include calcium, magnesium, and others that are dissolved in the water. You can’t usually see whether or not you have hard water just by looking at it, but you’ll probably be able to tell other ways. One way people quickly notice is that they tend to get “ring around the tub” and other mineral deposits in their sinks and bath tubs. You can also tell if you have hard water by how it lathers. Water with a lot of minerals won’t bubble up as much when soap is added. You can easily see the difference by comparing plain water with hard water that you mix yourself in this hard water experiment.
Step 1: Library Research
Before you begin experimenting, read a book or watch a video about water. Here are some suggestions, but if you can’t find these, look for books at your library or videos online about the properties of water. The colored smilies above each book or video tell you what age level they’re recommended for.
As Amazon affiliates, the recommended books and products below kick back a tiny percentage of your purchase to us. It doesn’t affect your cost and it helps us run our website. We thank you!

A Drop of Water
by Walter Wick


Properties of Water
by Amoeba Sisters

Water: A Polar Molecule
by Bozeman Science
Step 2: Hard Water Experiment
For this experiment you will need 2 plastic cups, a permanent marker, 2 straws, water (either already run through a water softener or distilled water), plaster of Paris, and dish soap.
Begin by labeling one cup “plain” and the other “hard” and then put soft or distilled water in each of the two cups. Stir a spoonful of plaster of Paris (calcium sulfate) into the cup labeled hard. It’s not entirely soluble, but that’s okay. It’s fine for some to sink down to the bottom of the cup. By adding calcium to the water you are creating your own hard water to test.

Add a few drops of dish soap into each cup and stir it in.
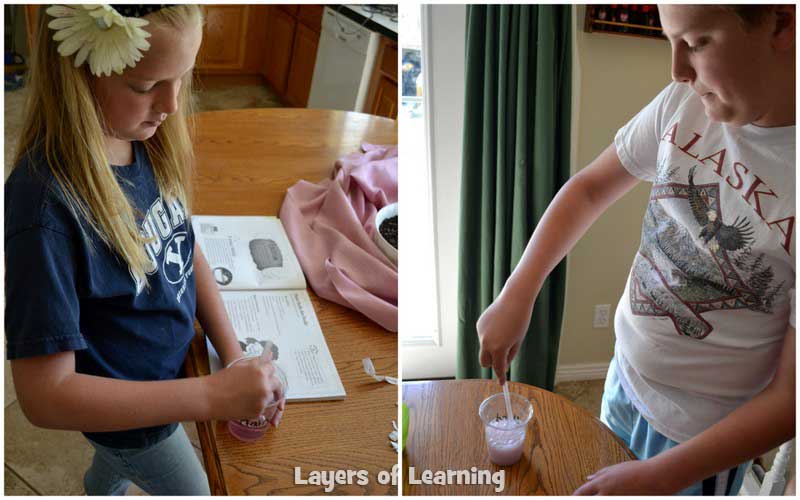
Now put a straw into each cup and begin gently blowing air into the water. Both will bubble up somewhat, but the soft water will produce more bubbles.

You can’t exactly tell from the pictures, but Tyler, with the hard water, worked much harder than Elizabeth to get his bubbles going.

The plain, soft water clearly suds more successfully.

Step 3: Show What You Know
Record the results of your experiment and write about what you’ve learned about water in your science notebook.
Additional Layers
Additional Layers are extra activities you can do or tangents you can take off on. You will find them in the sidebars of each Layers of Learning unit. They are optional, so just choose what interests you.
Fabulous Fact
Water molecules are strongly attracted to each other, also because of the positive and negative charges on opposite ends of the molecule.

This strong attraction between molecules creates surface tension and allows a paper clip to float on the surface.
Additional Layer
Most people prefer soft water for cleaning, but hard water for drinking. In the laundry, when doing dishes, when cleaning the bathroom, and even when cleaning ourselves, soft water is best. It works well with soap and doesn’t leave a nasty residue. However, most people prefer the taste of hard water. Try to drink distilled water. You probably won’t like it. It’s the minerals within water that make them taste good to us.
A lot of people have favorite brands of bottled water for this reason. You might think, “It’s just water. It’s all the same.” But that’s actually not true. It’s not the actual H2O flavor that we like in the bottled water; it’s the minerals within it.
Try doing a blind taste test of several brands of bottled water and see which you prefer.
Additional Layer
Things like calcium, dissolve easily in water because water is polar.

The negative and positive charges on each water molecule rip other ionic molecules apart.
Water is as close to a universal solvent (something that dissolves everything) as we’re likely to find.
Think about all the ways this dissolving power of water is useful in the natural world and in our bodies. Brainstorm a list together.
Get a Free Unit
Choose between the first unit in each Layers of Learning subject to try for free when you sign up for the newsletter.
We never spam and you can cancel your subscription at any time.








Hi Karen!
Thanks for sharing this experiment. I’ve noticed a huge difference in the amount of soap I need in the washer after getting a softener installed.
I noticed you mentioned that many people prefer hard water for drinking. Here in OKC the hardness of the tap water makes it unbearable to drink!
I much prefer the taste of soft water from the tap, but then again I also run my drinking water through my Brita before it hits my lips so perhaps that improves the taste of the soft water.
Thanks for taking the time to read my comment 😉
I like this test
Great