This exploration is for all ages, as the colored smilies show. You can do the floating eggs experiment with your whole family together!




The floating eggs is a physics experiment from the Fluids unit. Layers of Learning has hands-on experiments in every unit of this family-friendly curriculum. Learn more about Layers of Learning.
Density is how many molecules are packed into a space. The more molecules packed in, the more dense a substance is.
When salt is dissolved in water, it breaks down into ions that are attracted to the water molecules. This attraction causes them to bind tightly, increasing the amount of matter per volume (density). Instead of just having hydrogen and oxygen molecules in the water, sodium and chlorine joins in too(salt or NaCl). Salt water now has more particles in it compared to the plain tap water we started with; in other words, it’s more dense.
Step 1: Library Research
Before you begin exploring, read a book or two about density. Here are some suggestions, but if you can’t find these, look for books at your library about fluids or density. The colored smilies above each book tell you what age level they’re recommended for.
As Amazon affiliates, the recommended books and products below kick back a tiny percentage of your purchase to us. It doesn’t affect your cost and it helps us run our website. We thank you!

Things That Float and Things That Don’t
by David A. Adler

Step 2: Floating Eggs Experiment
You will need three clear cups or beakers, three eggs, water, and table salt. You can also use food coloring to make things brighter if you like.
First, carefully put an egg into a glass of warm water. If you like you can add food coloring to the glass of water just to make it prettier. The egg will sink because it is more dense than the water.
Next, make a saturated solution of salt and warm water. Stir salt in until you can’t stir in any more. When the salt is dissolved, put an egg in the salt water. You’ll see the egg rise to the very top because the egg is less dense than salt water. (That’s the middle glass in the picture.)

Finally, try a cool trick. Make the egg float in the middle of the water. Put warm salt water on the bottom (about 2/3 full). Then slowly and carefully pour in plain water on top. Gently put the egg in and watch it float between the 2 layers–above the salt water and below the plain water.


For Middle Grades and High School Only
To do the more advanced parts of this experiment, in addition to the above materials you will also need a scale and a beaker, which can measure liquid in milliliters.
For kids from about 10 years old and up you can also calculate the density of the eggs, the plain water, and the salt water. Before you do the calculations ask your kids to put the egg, plain water, and salt water in order from least dense to most dense according to the experiment you did above.
The equation to calculate density is mass divided by volume. Mass is measured in grams (g) and volume in milliliters (mL).
Density = mass/volume
So you need to measure the mass and the volume of the egg, the water, and the salt. Make a table to keep track of your calculations.

Measure the mass of the water by placing the beaker on a scale (or balance) and hitting “tare”. This will zero out the scale with the beaker on it so you are not measuring the mass of the beaker, only the mass of the water. Then pour water into the beaker to the 300 ml line. Record the mass in grams.


Measure the mass of the egg the same way. Put a container on the scale and hit “tare” then put the egg in the container and record the mass of the egg.
Do the same thing with the salt. Place a container on the scale and hit “tare” then pour salt into the cup and record the mass. For 300 ml of water you will need about 55-60 grams of salt to get a saturated solution.
The volume of the water is already recorded at 300 ml.
Find the volume of the egg by placing it in a carefully measured beaker of 300 ml of water. Note how much the water level rises and subtract 300 from the new volume to find the volume of the egg.


Measure the volume of the salt, the same way you did the egg, by adding the salt to 300 ml of water and measuring the new volume. Subtract 300 ml from the new volume.

Now start calculating
Calculate the density of the egg and the density of the water by dividing each mass by its volume.
To calculate the salt water you have to add the mass of the salt and the mass of the water. Then add the volume of the salt and the volume of the water. Then divide the added values.
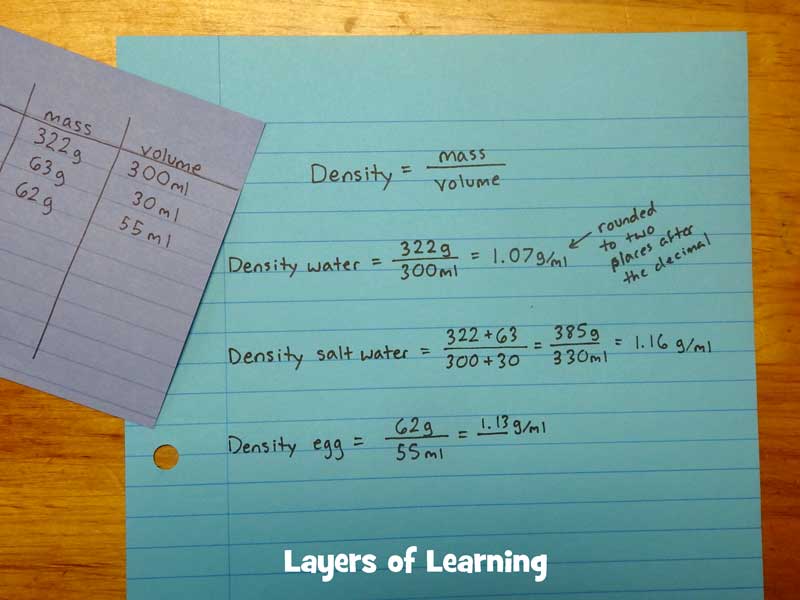
Now, according to your calculations, put the egg, water, and salt water in order from least dense to most dense.
You can calculate the density of any objects in this way. Try calculating the density of a Lego block, a metal toy car, a marble, a marshmallow, an air filled balloon, or anything other small items you have around the house.
Step 3: Show What You Know
Teach what you learned about density to someone who wasn’t there for the lesson, maybe Grandma, a neighbor, or Dad, for example. Show your student how to calculate density.
Additional Layers
Additional Layers are extra activities you can do or tangents you can take off on. You will find them in the sidebars of each Layers of Learning unit. They are optional, so just choose what interests you.
Memorization Station
Memorize the definition of density.
The quantity of mass per volume.
Also be able to explain what this means.
Fabulous Fact
Specific gravity is the density of a substance compared to water. Water is assigned the specific gravity of 1. Anything more dense than water will have a specific gravity of more than 1 and anything less dense than water will have a specific gravity less than 1.
Famous Folks
The ancient Greeks were the first to mathematically determine densities, volume and so on.

There is a story (maybe true, maybe not) that Archimedes discovered how to measure the volume of irregular objects when he was taking a bath and noticed the water level rise as he got in. “Eureka!” he exclaimed, which means “I’ve found it!” in Greek.
Get a Free Unit
Choose between the first unit in each Layers of Learning subject to try for free when you sign up for the newsletter.
We never spam and you can cancel your subscription at any time.









